Learn about RFA from Boston Scientific
Watch this short video to learn how radiofrequency ablation works.
Watch this short video to learn how radiofrequency ablation works.
RFA is a minimally invasive outpatient procedure. Your pain specialist targets pain-causing nerves and uses a radiofrequency current to interrupt the pain signals at their source.
Studies show that RFA provides effective, lasting relief from pain on suitable patients
Pain Relief
80% of patients from an international multicentred study reported a clinically significant reduction in pain with RFA.1
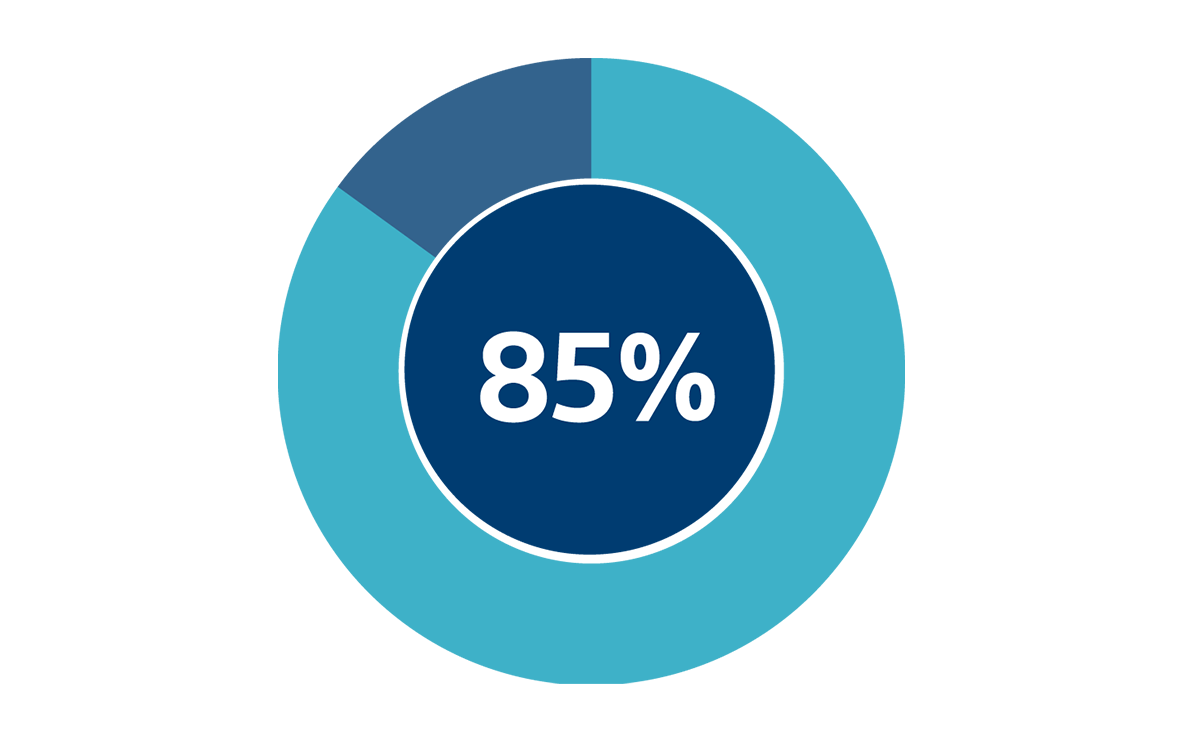
Patient Satisfaction
Over 85% of patients from a multicentred study reported being satisfied with the improvement in their symptoms.1
Lasting Relief
Patients reported continued pain relief at 12 months after their procedure.1 RFA procedures can be repeated to extend relief on suitable patients.2
References:
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
Disclaimer and endnotes:
Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.
Individual symptoms, situations, circumstances and results may vary. This quiz is meant for information purposes only, it is not intended to be used for medical diagnosis or treatment or as a substitute for professional medical advice. Please consult your doctor or qualified healthcare provider regarding your condition and appropriate medical treatment. This site is intended for Australian residents only. Please review the Boston Scientific Privacy Policy, for practices on the collection, storage, use and disclosure of your personal information.
Content of videos are for Information Purposes only and does not constitute medical advice. BSC strongly recommends that you consult with your physician on all matters pertaining to your health or to address any questions.
Talk to your healthcare professional about whether this product may be suitable for you as part of your overall plan to manage chronic pain. The WaveWriter Alpha System is not a first-line treatment for chronic intractable pain.
Surgery is required in order to use the WaveWriter Alpha System and any surgical procedure carries risk. Outcomes may vary for each patient. Patients should consult their healthcare professional about factors that could impact their response.
CAUTION: This product is not available for purchase by the general public. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com.
Australia and New Zealand: Boston Scientific Pty Ltd | PO Box 332 Botany NSW 1455 Australia